The
Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923),
is an acid-catalyzed rearrangement of an oxime to an amide. Most commonly used catalysts are Conc.H2SO4, HCl, PCl5, PCl3, SOCl2, ZnO, SiO2, PPA (Poly
phosphoric acid). Aldoximes are
less reactive than ketoximes. Cyclic
oximes yield lactams.
Mechanism of Beckmann
rearrangement:
Initially
the -OH group of the oxime is protonated. Then 1,2 shift of alkyl group (R1)
onto electron deficient nitrogen and the cleavage of N-O bond occurs
simultaneously.
Always the alkyl group which is 'anti' to the -OH group on
nitrogen undergoes 1,2 shift which indicates the concerted nature of the
beckmann rearrangement.
Where is it used?
This
reagent is useful in ring enlargement of cyclic ketone. A very good example is the industrial
conversion of cyclohexanone to caprolactam, which is used in the manufacture of
Nylon-6, involves Beckmann rearrangement
Mechanism
of this rearrangement is given below
Also
relative migratory aptitude comes in place when there are two different groups
as shown below.
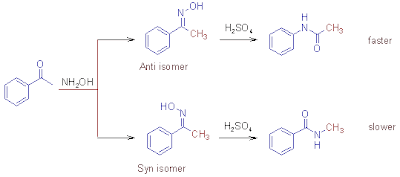
Above
two isomers for the unsymmetrical oxime are possible. When these oximes are rearranged mixture of products
are formed and the ratio in which they form is same as the isomer ratio in
oxime.
Oximes
derived from aldehydes are not good for Beckmann rearrangement because of the
poor yields for primary amides. Tosyl
chloride forms oxime tosylate which eliminates the stable tosylate anion. PCl5 and SOCl2 induce rearrangement
by converting OH to a better leaving group.
Certain
ketoximes (oximes of alpha-diketones, alpha-keto acids, alpha-dialkylamino
ketones, alpha-hydroxy ketones, beta-keto ethers) can be converted to nitriles
by the action of proton or Lewis acids via fragmentation reactions, which are considered
side reactions, often these are called as ‘abnormal’
or ‘second order’ Beckmann rearrangements.






A large part of Alfa Chemistry's customers are pharmaceutical and biotechnology companies, including Pfizer, Novartis, Merck & Co., Johnson & Johnson, AstraZeneca, and Bayer. Alfa Chemistry is also a preferred partner for many universities and non-profit institutes. 1-decyl-2,3-dimethylimidazolium tosylate
ReplyDelete